Discovery and Structure-Based Design of Potent Covalent PPAR gamma Inverse-Agonists BAY-4931 and BAY-0069 .
Orsi, D.L., Pook, E., Brauer, N., Friberg, A., Lienau, P., Lemke, C.T., Stellfeld, T., Bruggemeier, U., Putter, V., Meyer, H., Baco, M., Tang, S., Cherniack, A.D., Westlake, L., Bender, S.A., Kocak, M., Strathdee, C.A., Meyerson, M., Eis, K., Goldstein, J.T.(2022) J Med Chem 65: 14843-14863
- PubMed: 36270630
- DOI: https://doi.org/10.1021/acs.jmedchem.2c01379
- Primary Citation of Related Structures:
8AQM, 8AQN - PubMed Abstract:
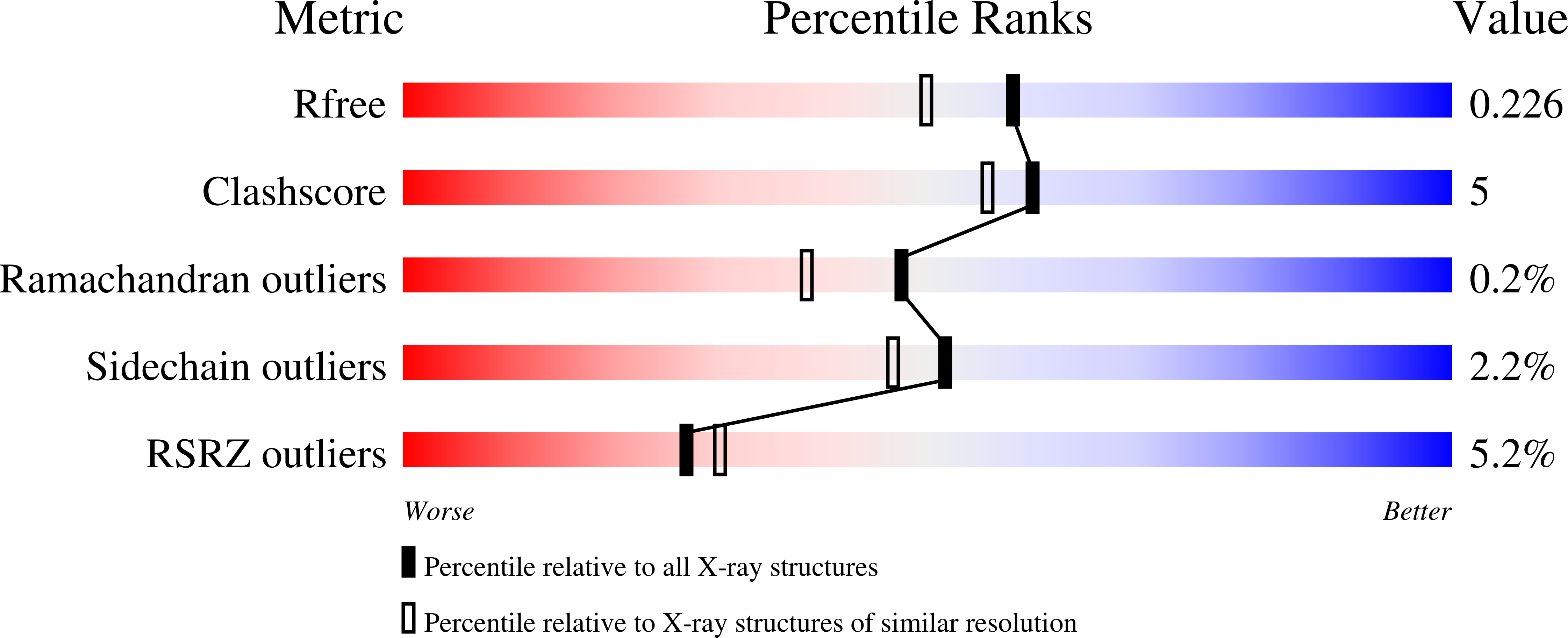
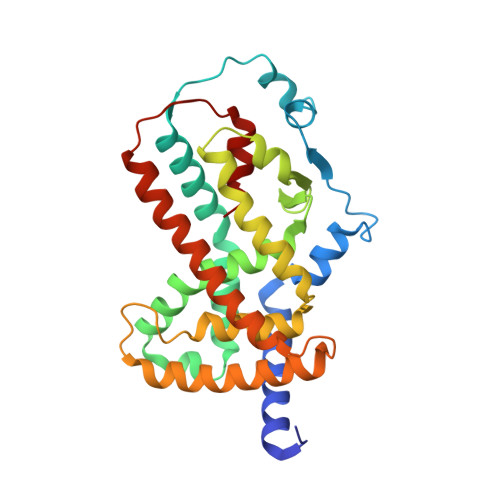
The ligand-activated nuclear receptor peroxisome-proliferator-activated receptor-¦Ã (PPARG or PPAR¦Ã) represents a potential target for a new generation of cancer therapeutics, especially in muscle-invasive luminal bladder cancer where PPAR¦Ã is a critical lineage driver. Here we disclose the discovery of a series of chloro-nitro-arene covalent inverse-agonists of PPAR¦Ã that exploit a benzoxazole core to improve interactions with corepressors NCOR1 and NCOR2. In vitro treatment of sensitive cell lines with these compounds results in the robust regulation of PPAR¦Ã target genes and antiproliferative effects. Despite their imperfect physicochemical properties, the compounds showed modest pharmacodynamic target regulation in vivo . Improvements to the in vitro potency and efficacy of BAY-4931 and BAY-0069 compared to those of previously described PPAR¦Ã inverse-agonists show that these compounds are novel tools for probing the in vitro biology of PPAR¦Ã inverse-agonism.
Organizational Affiliation:
Center for the Development of Therapeutics, Broad Institute of MIT and Harvard, Cambridge, Massachusetts 02142, United States.