Discovery of 2,6-difluorobenzyl ether series of phenyl ((R)-3-phenylpyrrolidin-3-yl)sulfones as surprisingly potent, selective and orally bioavailable ROR gamma t inverse agonists.
Duan, J.J., Jiang, B., Lu, Z., Stachura, S., Weigelt, C.A., Sack, J.S., Khan, J., Ruzanov, M., Wu, D.R., Yarde, M., Shen, D.R., Zhao, Q., Salter-Cid, L.M., Carter, P.H., Murali Dhar, T.G.(2020) Bioorg Med Chem Lett 30: 127441-127441
- PubMed: 32736080
- DOI: https://doi.org/10.1016/j.bmcl.2020.127441
- Primary Citation of Related Structures:
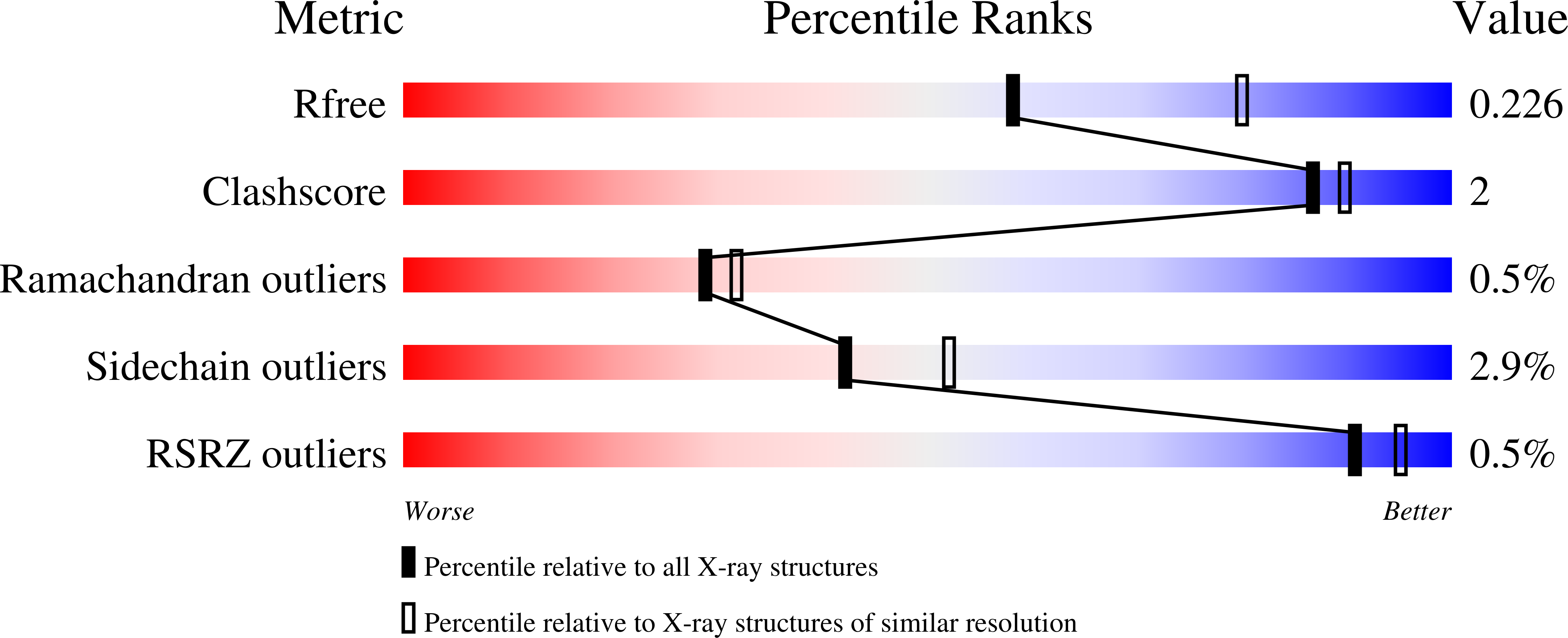
7JH2 - PubMed Abstract:
In an effort to discover oral inverse agonists of ROR¦Ãt to treat inflammatory diseases, a new 2,6-difluorobenzyl ether series of cyclopentyl sulfones were found to be surprisingly more potent than the corresponding alcohol derivatives. When combined with a more optimized phenyl ((R)-3-phenylpyrrolidin-3-yl)sulfone template, the 2,6-difluorobenzyl ethers yielded a set of very potent ROR¦Ãt inverse agonists (e.g., compound 26, ROR¦Ãt Gal4 EC 50 11?nM) that are highly selective against PXR, LXR¦Á and LXR¦Â. After optimizing for stability in human and mouse liver microsomes, compounds 29 and 38 were evaluated in vivo and found to have good oral bioavailability (56% and 101%, respectively) in mice. X-ray co-crystal structure of compound 27 in ROR¦Ãt revealed that the bulky benzyl ether group causes helix 11 of the protein to partially uncoil to create a new, enlarged binding site, which nicely accommodates the benzyl ether moiety, leading to net potency gain.
Organizational Affiliation:
Research and Early Development, Bristol Myers Squibb Company, Princeton, NJ 08543-4000, United States. Electronic address: james.duan@bms.com.